by Justine de Valicourt
Overview
Cyanide is a strongly toxic compound that is frequent in nature, including in some plants generally considered safe. We investigated its pathway to have a better understanding of its toxicity. A lot of food contains small concentrations of cyanide in the form of glycosides. When the plant is hurt or chewed a chemical reaction occurs in the harmed cell and the cyanide is released as a natural defense. This poison blocks the cell’s uptake of oxygen, bringing on a cellular condition called histotoxic anoxia, lit. ‘cell toxic lack of oxygen’. The cell is no longer able to produce energy for its normal functions and quickly dies.
The human body can detoxify a small amount of cyanide in the liver through a pathway involving a molecule called thiosulfate. Poisoning occurs when there is not enough thiosulfate to neutralise all the cyanide. At low toxic concentrations the cyanide can provoke nausea, vomiting, general weakness and dizziness. A lethal dose to humans is thought to be 98mg in one day, with a lowest documented lethal dose of 37.8mg.
Cyanide has a boiling point of 25.7°C, which means that even a little heat would vaporise the toxin and make the product safer.
We focused our investigation on the cyanide content of black elder and elderberries. Elder (Sambucus nigra) leaves, bark, roots, unripe fruits and stems contain high levels of cyanide. Completely ripe fruits are generally considered safe to eat. Scientific literature has related few cases of food poisoning from elderberries, but each event was probably brought on by the accidental use of leaves and stems along with the berries.
To satisfy our curiosity on the subject of cyanide, we decided to prepare a common semi-quantitative test to evaluate the level of cyanide in food. The test uses the reaction of picric acid (yellow compound) changing into isopurpuric acid (brown-reddish) in the presence of volatile hydrogen cyanide (HCN). The HCN contained in any solution turns the paper from bright yellow to brick orange. The intensity of the colour change gives a rough idea of the concentration of HCN. A more refined test would be to make a scale of colour with known concentrations of HCN, and then to compare picric acid paper analyses from food products with the colour scale to approximate the food’s HCN concentration.
Elderflower season is over. Their magnificent perfume is no longer around us. We made good use of the season, however, harvesting it wherever we found it. Our different projects involving elderflower have made us think about the safety of consuming the elder plant, its flowers and berries. We knew that elder can be toxic, but were not sure about the circumstances of this toxicity or how best to remove it. Elder, along with other plants like stone fruits and cassava, contains hydrogen cyanide (HCN) in the form of cyanogenic glycosides. This usually inactive molecule is broken down by enzymes when the plant is harmed, or the pit crushed, and hydrogen cyanide is released as a natural repellent against insects and other hungry predators.
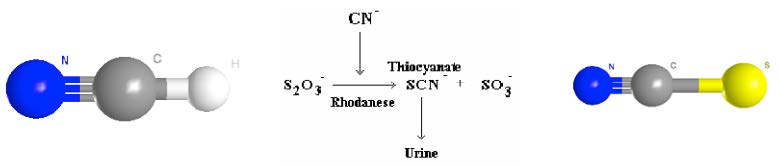
So we began researching toxicity and human tolerance to HCN. It seems that an ‘average’ human could be killed by ingesting 70g of apple seeds. That is a lot of apple in one day. In fact, the body is able to detoxify small amounts of HCN in the liver, through an enzyme called rhodanese [1] that uses a molecule of thiosulfate to bind the cyanide group and render it harmless. The resulting thiocyanate is excreted in the urine, while the free sulphate group is available for another purpose. Poisoning from HCN thus occurs when there is not enough thiosulfate available to neutralise the cyanide.
The Electron Transport Chain
Yet why is cyanide dangerous in the first place? The molecules enter the cell, blocking the complex reaction that transforms oxygen into energy: the electron transport chain.
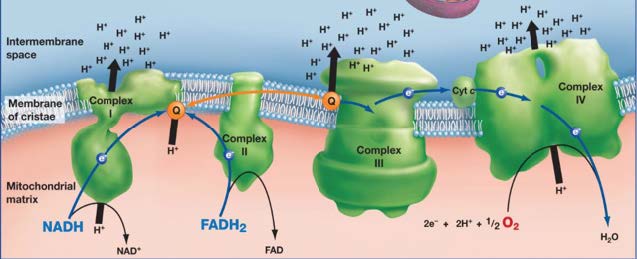
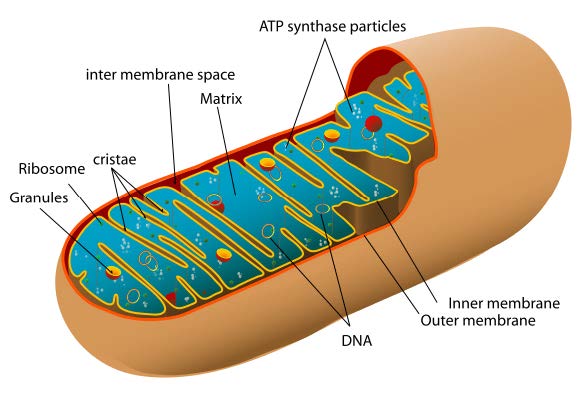
This chain of enzyme complexes is situated in an organelle called the mitochondrion. The mitochondria have a particular structure: they are composed of two membranes – unlike other organelles which have just one – which create an intermembrane space in addition to the inner mitochondrial matrix.
The four types of enzyme complex are attached to the inner membrane of the mitochondria and permit a gradient of electrons to develop between the inner space and the intermembrane space. This gradient is necessary for the final step in the transformation of adenosine diphosphate (ADP) into adenosine triphosphate (ATP) by a fifth enzyme called ATP synthase. ATP synthase attaches a third phosphate group onto ADP using the energy from the electrochemical gradient and the influx of H+ ions. ATP is the body’s chemical energy – almost every metabolic reaction needs it. It is called a co-enzyme because most other enzymes cannot work properly without it. ATP is the currency of the body’s chemical economy.
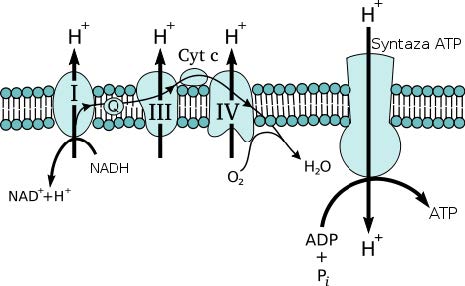
The cyanide molecule intervenes in this process, fixing itself to the cytochrome c oxidase, the main enzyme of the fourth enzyme complex, and changing its configuration. This alteration makes it impossible for the complex to transfer H+ ions to the intermembrane space and to synthesise them into water with oxygen. By blocking this crucial step, the cyanide effectively disrupts the entire electron transport chain. ATP is no longer produced in the aerobic conditions and the cells die quickly from a condition called histotoxic anoxia (cellularly toxic lack of oxygen). The first cells to be harmed by the poison are those in organs that cannot perform under anaerobic conditions: first the brain, then the liver and the other vital organs.
Death by apples
The scientific literature relates a few cases of poisoning from fruit consumption: children dying from eating apricot and cherry pits [5]; a women who became poisoned by drinking choke cherry spirit [6]. In the latter case, the spirit and cherries were analysed for HCN content with results of 43-45mg/kg and 4.7-15mg/kg respectively. Although it is quite impracticable to study a lethal dose for humans without killing any, some studies have proposed a potential lethal dose of 1.4mg per kg of body weight, with a lowest lethal dose documented of 0.54mg per kg of body weight1. For an average man of 70kg, this means an ingestion of 37.8mg in one day.
The following table presents different food products and their concentration of HCN [1].
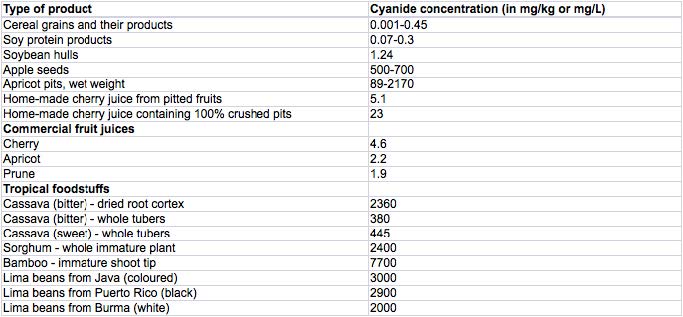
The crucial fact here is that HCN has a boiling point of 25.7°C. Which means that in preparations involving heat, and even a slight incubation above room temperature, most of the free cyanide will evaporate, leaving the food product much safer.
Beyond these few clear facts, the literature holds a lot of confusion. All sources agree that the elder tree has cyanogenic glycosides and thus the potential for HCN; yet some sources suggest the berries and flowers are safe to eat, some say not really, and some say they should always be heated before consumption. Furthermore, we haven’t found any article that clearly states a concentration of HCN in either the berries or the flowers. Which brings us back to our central question: are elderberries safe to eat raw? In what quantities? Do they harbour other toxins that could be harmful when ingested?
A relevant project of ours is an elder vinegar, using elderflower wine and adding the berries before the acetic fermentation (another post on this will come soon). Will this vinegar be safe to consume in large quanities?
After a bit of digging we found that raw elderberries contain 3mg of potential HCN (in the form of glycosides) per 100g of fruit, making them dangerous for cyanide poisoning only with a consumption of more than 1.5kg of raw fruit in a day. While that’s quite a bit of berries, it’s not out of the realm of possibility – though when we’re talking about vinegar, it may be safe if for no other reason than that one rarely drinks a bucketful. Toxicity is always about dose and concentration.
That said, we like to have more precise answers and to be able to see them for ourselves. Through our research we discovered it is quite easy to make our own test to determine the HCN content of any product.
The DIY picric acid test for HCN
Pure picric acid is an explosive. But in solution, it is quite harmless (for an acid). Picric acid itself is bright yellow, but when it reacts with cyanide it is reduced to a more orange brown compound call isopurpuric acid [7]. By soaking filter paper in picric acid, one can make simple test paper that gives slightly-better-than-binary results. Once dry, the paper will react with HCN vapour, which in practice means that if you put a cyanogenic product in a test tube with some water to soak it, then suspend a strip of this paper at the top of the test tube before closing it, the vapour from the product will interact with the picric acid and make the colour of the paper changes from a bright yellow to an orange-brown. The more cyanide in the product, the browner the paper.
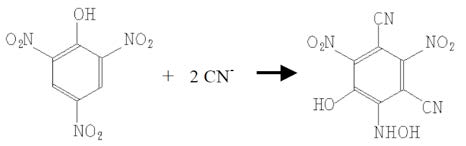
We made an initial test with cassava to calibrate our paper, as cassava has one of the highest concentrations of HCN in a food product. Here are the results:
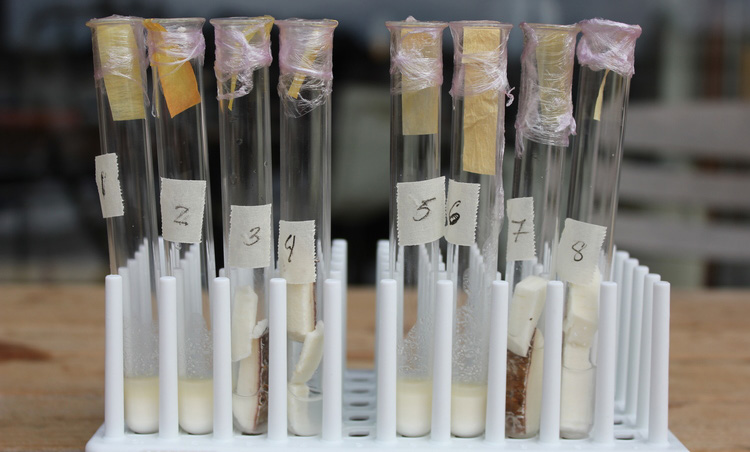
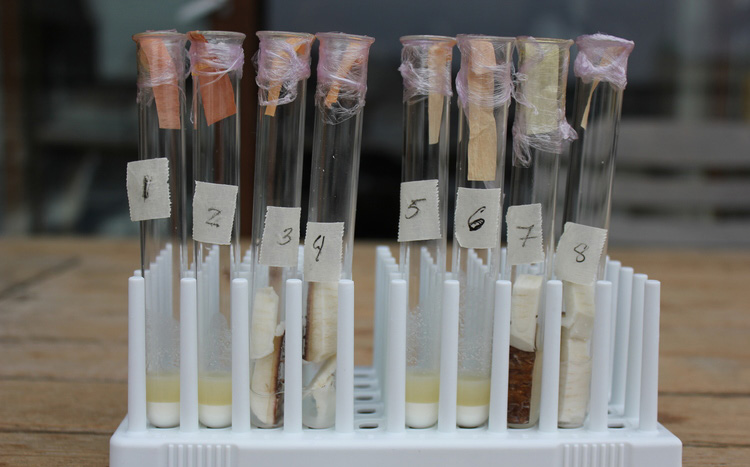
It works.
The next step is to make graded test strips with solutions of different concentrations of sodium cyanide (NaCN). We will then have a semi-quantitative test because we will be able to use the relative concentration of cyanide in NaCN as a benchmark for comparing the HCN concentration of many different foods. Hopefully we can get it ready before the elder vinegar!
[addendum]
While the HCN in elderberries can poison one to death, it became clear through our investigation that it was not the only source of the common emetic and purgative symptoms of consuming the berries raw. We’ve since looked further into elder, with more findings to come.
[erratum 2.11.13]
Thanks to Julia Stasinska for spotting an error in the table – 5mg/kg of cyanide in apple seeds is actually 500-700mg/kg.
Bibliography
1 Concise International Chemical Assessment Document 61, HYDROGEN CYANIDE AND CYANIDES: HUMAN HEALTH ASPECTS
2 Wiki Tox, Open Source Clinical Toxicology Curriculum. 2.2.9.1.2 Cyanide. Online. http://curriculum.toxicology.wikispaces.net/2.2.9.1.2+Cyanide
3 University of Illinois at Chicago. Glycolysis, Krebs Cycle, and other Energy-Releasing Pathways. Online. http://www.uic.edu/classes/bios/bios100/lectures/respiration.htm
4 Wikimedia Commons. Mitochondrial electron transport chain short PL.svg. Online. http://commons.wikimedia.org/wiki/File:Mitochondrial_electron_transport_chain_short_PL.svg
5 Sayre JW, Kaymakcalan S (1964) Cyanide poisoning from apricot seeds among children in Central Turkey. New England Journal of Medicine, 270:1964.
6 Nahrstedt AF (1993) Cyanogenesis and food plants. In: van Beek TA, Breteler H, eds. Proceedings of the International Symposium on Phytochemistry and Agriculture, 22–24 April 1992, Wageningen. Oxford, Oxford University Press, pp. 107–129.
7 The Picric Acid Method for Determining Weak Acid Dissociable (WAD) Cyanide, published by MEP Instruments and Applicon Analyctical®. Online. http://curriculum.toxicology.wikispaces.net/2.2.9.1.2+Cyanide
