posted by Jonas Astrup Pedersen
Kombucha, also known as Kargasok Tea, Tea Fungus, Haipao and Manchurian Mushroom, is a fermented beverage dating back several thousand years in the East. More recently, it has become popular in the West, specifically in ‘New Age’ circles (Battikh et al., 2012; Jarrell et al., 2000; Greenwalt et al., 2000). Tea fungus initially originated in China in 220 BCE during the Tsin Dynasty and prized as the ‘Divine Che’. The name ‘Kombucha’ seems associated with Doctor Kombu, who is said to have brought the ‘tea fungus’ from Korea to Japan in 414 CE (Dufresne and Farnworth, 2000).
Increasing interest in Kombucha products is linked to their supposed therapeutic benefits, ranging from curing cancer and AIDS to enhancing weight loss, as well as demonstrating interesting sensory properties (Dufresne and Farnworth, 2000; Teoh et al., 2004). Although several of these claims are not proven, Kombucha beverages exerts antimicrobial activity against Salmonella typhimurium, Staphylococcus aureus, Helicobacter pylori, (Greenwalt et al., 1998), Shigella sonnei, Salmonella enteritidis and Escherichia coli (Greenwalt et al., 1998; Sreeramulu et al., 2001). Furthermore, Kombucha tea ingestion by mice contributed significantly to both life elongation and weight gain inhibition (Hartmann et al., 2000).
The beverage is typically made with black tea, sweetened with 5 to 15% of sucrose, and set to ferment at room temperatures for 10-12 days with a culture popularly known as a ‘tea fungus’, Medusomyces gisevii (Anken and Kappel, 1992; Jayabalan et al., 2010). Inoculation of new batches uses about 10% of Kombucha from a previous batch. The brewing vessel is covered with a clean cotton cloth to keep out debris while allowing aeration (Greenwalt et al., 2000). A schematic description of Kombucha production is seen in Figure 1.
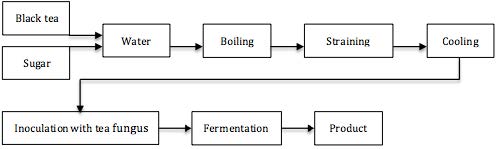
Kombucha is the expression of a symbiotic growth of bacteria such as Acetobacter xylinum, A. xylinoides, A. aceti, A. pasteurianus, Bacterium gluconicum (Sreeramulu et al., 2000; Dufresne and Farnworth, 2000) and yeasts like Schizosaccharomyces pombe, Kloeckera apiculata, Saccharomycodes ludwigii, Saccharomyces cerevisiae, Zygosaccharomyces bailii, Brettanomyces bruxellensis, B. lambicus, B. custersii and Pichia species (Dufresne and Farnworth, 2000). Though the fungus-like cellulosic matrix produced by especially Acetobacter xylinum might look like a fungus (Mo et al., 2008), ‘tea fungus’ is rather misleading since the ‘tea fungus’ is in fact only a physical manifestation of the yeast and bacteria symbiosis (Sreeramulu et al., 2000). The floating jelly-like membrane, called a zoogleal mat, is where the cell mass of the bacteria and yeasts are attached (Jayabalan et al., 2010). The cellulose is a secondary metabolite of the fermentation, similar in structure to a ‘mother of vinegar’ (Jayabalan et al., 2010). Within the cellulose network, investigations have shown the Kombucha colony to be arranged in bands and layers (Anken and Kappel, 1992), see Picture 4. The composition and exact diversity of the microbiological presence depends on the source of the Kombucha culture (Sreeramulu et al., 2000).
As yeast cells hydrolyze sucrose into glucose and fructose, producing ethanol and carbon dioxide as metabolites, acetic acid bacteria converts glucose into gluconic acid and fructose into acetic acid (Reiss, 1994; Loncar et al., 2006). The primary metabolites of ethanol and acetic acid behave as catalyzing agents; yeast are stimulated to produce ethanol by acetic acid, whereas ethanol stimulates the growth of acetic acid bacteria and their production of acetic acid (Liu et al., 1996). Fructose is utilized to a lesser degree and remains part of the fermented liquid (Greenwalt et al., 1998). The synthesis of complex B vitamins and folic acids has also been reported during the fermentation process (Bauer-Petrovska and Petrushevska-Tozi, 2000). Additionally, the organic acids produced throughout the fermentation and the corresponding decrease in pH value prevent the symbiotic culture from becoming contaminated by undesirable microorganisms not contained in the tea fungus (Greenwalt et al., 1998; Mo et al., 2008).
The properties and composition of the final product depends on the initial substrates, to geographical and climatic conditions, as well as the locally-specific types of wild yeast and bacteria present (Bauer-Petrovska and Petrushevska-Tozi, 2000). To obtain beneficial attributes and antimicrobial activity against a range of pathogenic bacteria, Greenwalt et al. (1998) recommends consumption of Kombucha containing 33 g/L total acids, 7 g/L acetic acid. Usually, the pH of a fermented Kombucha is around 2.5, regarded by the food industry as a high-acid food since a pH of 4.0 prevents growth of most organisms linked with spoilage (Greenwalt et al., 2000).
Experiments
Herbs or wood and boiling water (1 L each) were added to separate containers, closed with a lid and left to infuse at room temperature with different infusing times for optimal flavour intensity. These included dried yarrow flowers (1% w/v, 10 min.), juniper wood (5% w/v, 1 hour), dried chamomile (1% w/v, 10 min.), dried lemon verbena (1% w/v, 10 min.), dried woodruff (1% w/v, 10 min.) and dried cèpes (5% w/v, 12 hour). Dried kelp (2.3% w/v, 1 hour) was sealed in a vacuum bag and treated sous vide at 60 °C. To all solutions were added 50 g of sucrose.

The Kombucha mothers were carefully cut into approximately equal sizes and added to the teas. Additionally, 100 mL (10% v/v) of the liquid medium (tea kvass) were also added. Containers were covered with a cloth and set into a closed cabinet at an ambient temperature of approximately 21 ± 2 °C.
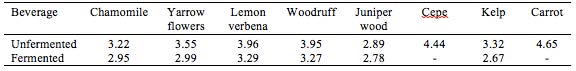
Based on sensory evaluation among employees at Nordic Food Lab (NFL) during the period of fermentation and at day 12, lemon verbena (Aloysia triphylla) was selected as a basis for further elaboration. The absence of data for cepe and carrot is due to loss of samples to mold, most likely because of too high initial pH.
To produce sufficient quantity of the lemon verbena Kombucha for further investigation, a batch of 17 L of tea was brewed: dried lemon verbena (1% w/v, 10 min.) was steeped in boiling water, to which was added 50 g of sucrose per liter. The batch was inoculated with tea kvass (0.95 mL) from the previous batch. The container was put into an incubator box to control fermentation temperatures, which measured in the range of 27 ± 2 °C.

Lemon verbena Kombucha
The herb mentioned as lemon verbena (Aloysia triphylla (L’Hérit.) Britt. Syn. Lippia citriodora) belongs to the family of Verbenáeae (in Danish: jernurt-familien) (Vogel et al., 1999). It is well known for the pleasant odour of its leaves, comparable to that of a lemon. Responsible for its aromatic properties are essential oils found in concentrations of 0.4% (Montes et al., 1973) to 1.2% (Vogel et al., 1999). Its pronounced lemon-like odour is due to the chemical compound citral found in concentration of lemon verbena oils between 11% and 54% (Montes et al., 1973; Vogel et al., 1999).
Lemon verbena, also called cedrón in its countries of origin, is a shrub native to Peru, Chile and Argentina where it is cultivated for domestic consumption as an herb tea (Vogel et al., 1999). It was brought to Europe during the 18th century and grown as potted plants due to its high sensitivity to cold (Vogel et al., 1999). Despite its origin in South America, lemon verbena has caught the attention of chefs and is now found in many Nordic kitchens and dishes.
In cooking circles, an often-heard misunderstanding of its name is the simple use of (in Danish) jernurt. Jernurt refers merely to the family Verbenáeae, which contains some 25 34 genera and 500-1200 species comprising a great variety of small trees, lianas, shrubs and herbs (Yuan et al., 2010).
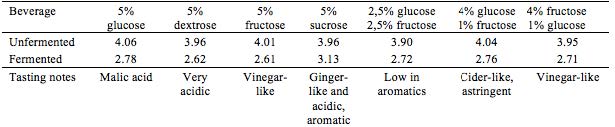
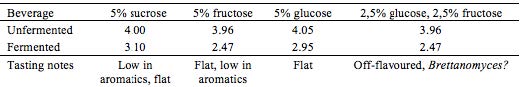
In an attempt to speed up the fermentation process, aeration was tested on four brews. An aquarium pump (AM-TOP model CR10) was connected to the containers during the entire fermentation process. Different kinds of sugar, either ‘alone’ or in combination, were tested as different energy sources for the yeast and bacteria. This was done with the purpose of observing and detect possible variation in sensory qualities and pH of end products. Teas were prepared as in the preliminary trials: herb extraction (1% w/v, 10 min.), added sugar (5% w/v) and finally inoculation with tea kvass (10% v/v) at 21 ± 2 °C, see Table 2 and Table 3.
Where fructose is stated, Danish honey from Søborg was used.
Results
With the aerated batches, enhancing the fermentation speed did not seem to succeed in favouring the acetic acid bacteria. Of the four batches, none were as balanced, as complex in flavor, or as refreshing as Kombucha can be. Although Kombucha is acidic, this tartness was not satisfying when complementary aroma was absent or expressed in a flat manner.
Testing different sugars revealed great difference in end product. Some Kombuchas turned out almost vinegar-like and not appropriate for a soft drink, though these could have interesting applications for culinary exploration and use. By far the most aromatic and interesting Kombucha developed in this experiment, contained sucrose as the sugar substrate. It expressed herby notes as well as an interesting ginger-like association, well balanced, pleasantly acidic and complex.
Alongside these small-batch trials, a continuous batch has served as staff Kombucha. Sweetened lemon verbena tea has been added, throughout the project, to a large vessel used during the process, while Kombucha has been tapped and enjoyed. The Kombucha turned out very tasty in expression: fizzy, refreshing, and especially delicious when served ice-cold. This encourages further investigation into continuous fermentations with e.g. higher levels of inoculum to amount of sweetened tea, as well as second fermentations in terms of added juice or other flavourfull sugar containing liquids, is a path believed worth investigating. KOMBOOOUCHA!
Literature
[1] ANKEN, R. H. & KAPPEL, T. 1992. Histochemical and anatomical observations upon the tea fungus, Liége, Belgique, Vaillant-Carmanne.
[2] BATTIKH, H., BAKHROUF, A. & AMMAR, E. 2012. Antimicrobial effect of Kombucha analogues. LWT – Food Science and Technology, 47, 71-77.
[3] BAUER-PETROVSKA, B. & PETRUSHEVSKA-TOZI, L. 2000. Mineral and water soluble vitamin content in the Kombucha drink. International Journal of Food Science and Technology, 35, 201-205.
[4] DUFRESNE, C. & FARNWORTH, E. 2000. Tea, Kombucha, and health: a review. Food Research International, 33, 409-421.
[5] GREENWALT, C. J., LEDFORD, R. A. & STEINKRAUS, K. H. 1998. Determination and Characterization of the Antimicrobial Activity of the Fermented Tea Kombucha. LWT – Food Science and Technology, 31, 291-296.
[6] GREENWALT, C. J., STEINKRAUS, K. H. & LEDFORD, R. A. 2000. Kombucha, the fermented tea: Microbiology, composition, and claimed health effects. Journal of Food Protection, 63, 976-981.
[7] HARTMANN, A. M., BURLESON, L. E., HOLMES, A. K. & GEIST, C. R. 2000. Effects of chronic kombucha ingestion on open-field behaviors, longevity, appetitive behaviors, and organs in c57-bl/6 mice: a pilot study. Nutrition, 16, 755-761.
[8] JARRELL, J., CAL, T. & BENNETT, J. W. 2000. The Kombucha consortia of yeasts and bacteria. Mycologist, 14, 166-170.
[9] JAYABALAN, R., MALINI, K., SATHISHKUMAR, M., SWAMINATHAN, K. & YUN, S.-E. 2010. Biochemical characteristics of tea fungus produced during kombucha fermentation. Food Science and Biotechnology, 19, 843-847.
[10] LIU, C. H., HSU, W. H., LEE, F. L. & LIAO, C. C. 1996. The isolation and identification of microbes from a fermented tea beverage, Haipao, and their interactions during Haipao fermentation. Food Microbiology, 13, 407-415.
[11] LONCAR, E., DJURIC, M., MALBASA, R., KOLAROV, L. J. & KLASNJA, M. 2006. Influence of Working Conditions Upon Kombucha Conducted Fermentation of Black Tea. Food and Bioproducts Processing, 84, 186-192.
[12] MO, H., ZHU, Y. & CHEN, Z. 2008. Microbial fermented tea – a potential source of natural food preservatives. Trends in Food Science & Technology, 19, 124-130.
[13] MONTES, M., VALENZUELA, L., WILKOMIRSKY, T. & ARRIVÉ, M. 1973. Sur La Composition De L’Essence D’Aloysia triphylla (“Cedron”). Planta Med, 23, 119-124.
[14] REISS, J. 1994. Influence of different sugars on the metabolism of the tea fungus. Zeitschrift Fur Lebensmittel-Untersuchung Und-Forschung, 198, 258-261.
[15] SREERAMULU, G., ZHU, Y. & KNOL, W. 2000. Kombucha Fermentation and Its Antimicrobial Activity. Journal of Agricultural and Food Chemistry, 48, 2589-2594.
[16] SREERAMULU, G., ZHU, Y. & KNOL, W. 2001. Characterization of antimicrobial activity in Kombucha fermentation. Acta Biotechnologica, 21, 49-56.
[17] TEOH, A. L., HEARD, G. & COX, J. 2004. Yeast ecology of Kombucha fermentation. International Journal of Food Microbiology, 95, 119-126.
[18] VOGEL, H., SILVA, M. L. & RAZMILIC, I. 1999. Seasonal flcutuation of essential oil content in lemon verbena (Aloysia triphylla), Mendoza, Agentina
[19] YUAN, Y.-W., LIU, C., MARX, H. E. & OLMSTEAD, R. G. 2010. An empirical demonstration of using pentatricopeptide repeat (PPR) genes as plant phylogenetic tools: Phylogeny of Verbenaceae and the Verbena complex. Molecular Phylogenetics and Evolution, 54, 23-35.
