by Guillemette Barthouil
Overview
Smoking is valued nowadays not only for preserving food, but also as a distinct set of flavours that can reflect the landscape of the region. Pyrolysis, the thermochemical decomposition of organic matter, breaks apart the three main components of wood: cellulose, hemicellulose and lignin. Each influences the colour, flavour, preservation and surface texture of the food – this information is summarized in Figure 4 below. Controlling pyrolysis is the key know-how involved in good smoking. Overall, for best results the wood moisture should be lower then 25% and its combustion temperature around 400°C.
“Smoking is one of the oldest food preservation methods, probably having arisen shortly after the development of cooking with fire.” (Encyclopedia Brittanica)
Most articles I have read for this research have started with the same statement. Humanity has used this technique for so long, it seems rather strange to try to explain it – but that is our job. All chefs know one has to understand a product or technique in order to make the most out of it. We use some scientific knowledge to enhance the desired, latent characteristics of a product, with the goal of better understanding how traditional knowledge could have been cultivated in a geographic area. And smoking is a great example.
The food history of the Nordic countries is particularly characterised by a ‘storage economy’ – a concept applicable to most parts of the world with seasons, here emphasised by the extreme climate. Most processes that we study -– drying, salting, smoking, fermenting, and others – were indispensable in these conditions. By increasing heat and lowering humidity, smoking enabled food to dry up more quickly, preserving it for long periods of time.

In the past decades, the taste for salted, dried and fermented food has shifted toward fresher food, gravlax being a good example. The taste for smoked products, however, has remained. Smoking is now not only important for preserving food, it is a valued flavor that can reflect the landscape of the region. Smoking in time and place depends on many things: the seasonal fish available, the smoking technique and temperature used – for cold smoking, the temperature drops the further north you go – and most importantly, the type of wood. Trees are part of the landscape before they become an essential component of the product’s taste. Alder trees growing in humid areas have been used along the west coastline of Norway and on the island of Bornholm in the Baltic; birch is a typical flavor from mountainous zones; dried manure is still used in the windy and harsh Icelandic land since trees and shrubs are in short supply; this list could go on. Understanding these traditions allows us to think of other flavors that could emerge from a specific land as we come to know it. Gaining a deeper understanding of wood pyrolysis and the science of smoke will also help us in our choices.
Smoke is not a simple gas. It is a mixture of three state of matter: an aerosol of solid particles, liquid drops and vaporized chemicals. These vaporized chemicals only count for 10% of the volume but do more then 90% of the job. And as you might have guessed, the composition of the smoke depends on the nature of the burning fuel and the conditions of combustion.
Pyrolysis is a thermochemical decomposition of organic matter brought about by high temperatures. To produce quality smoke the wood should undergo an incomplete combustion of organic materials in the presence of limited oxygen and medium temperature.
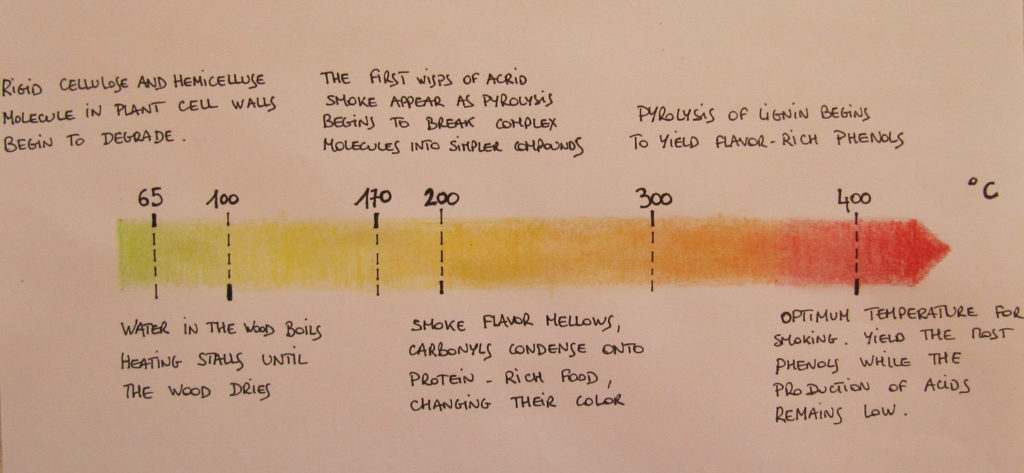
Wood consists of three primary materials. The wood’s cell wall is composed of micro-fibrils of cellulose (40 to 50%) and hemicellulose (15 to 25%) impregnated with lignin (15 to 30%).
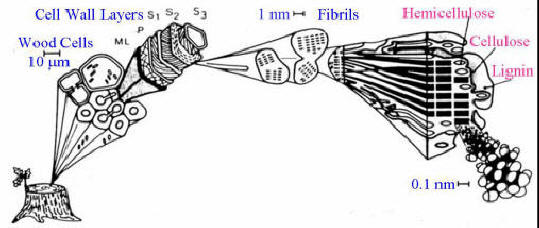
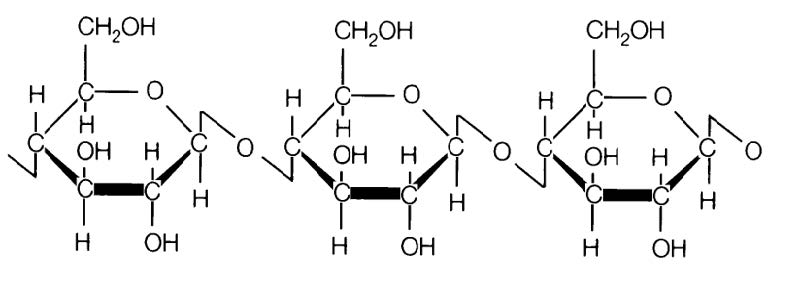
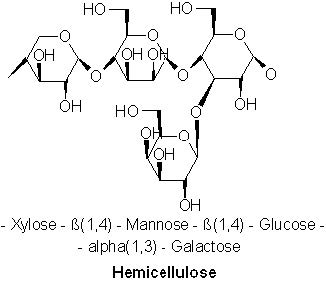
Hemicellulose is a matrix of polysaccharides present along cellulose is almost all plant walls. It differs from cellulose in that hemicellulose is a shorter chain branched polymer with low molecular weight. While cellulose is composed only of glucose, hemicellulose can include other sugars like xylose, mannose (both found predominantly in hardwood trees), galactose (in softwood trees), rhamnose and arabinose. Though both aggregates of different sugars, cellulose and hemicellulose break down into similar molecules during the incomplete combustion.
Lignin provides compressional strength to the cell wall, unlike the flexible strength conferred by cellulose. Without lignin, terrestrial plants probably could not have reached the sizes they do today, as cellulose by itself does not provide enough resistance to gravity. It is made of intricately interlocked phenolic molecules – essentially rings of carbon atoms with various additional chemical group attached – and its one of the most complex known natural substances. The higher the lignin content of wood, the harder it is and the hotter it burns: its combustion releases 50% more heat than cellulose.
The sugar in cellulose and hemicellulose breaks apart into many of the same molecules found in caramel. They are carbonyl molecules that react with amino acids and sugars to create a Maillard-type reaction that generates new flavours and yellow to dark brown color. During pyrolysis, sweet maltitols, bread-like aroma from furans, nutty lactones, and other volatile molecules are produced. All together they soften the heavy phenolic compounds.
The interlocked phenolic rings of lignin break apart from each other into smaller, volatile phenols and other molecules which most often have specific aromas. The most distinctive ones are isoeugenols, one of the main flavour component of clove, creosols that bring peat notes, vanillin that smells as it sounds, guaiacols contributing to the general spiciness.
The smoke has many other effects due to many different chemicals broken apart during the pyrolysis. The following table tries to summarise them.
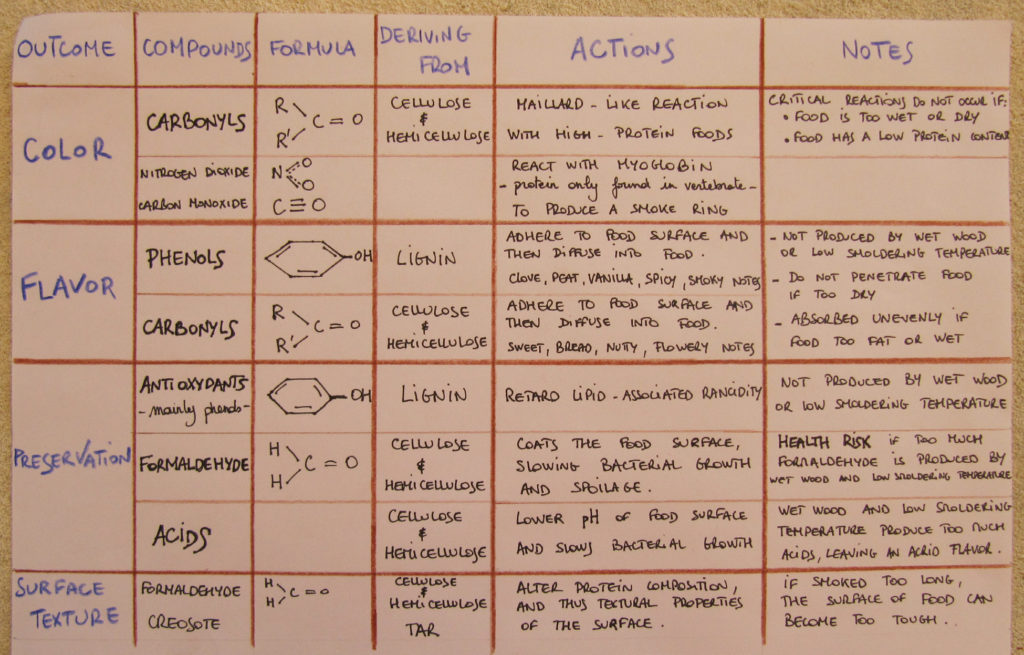
As you might have noticed in the ‘notes’ column, capturing the desired smoking effects depends mainly on the wood humidity and the smouldering temperature. Controlling the pyrolysis is the key know-how of a good smoker. Freshly-cut wood contains 40-60% moisture which is not suitable for smoking. A good wood containing less than 25% moisture is preferred. Professionals agree that the combustion temperature is best around 400°C. Higher than this, the flavour molecules are broken down into simpler, harsh, or flavourless molecules. Lower pyrolysis – under 200°C – degrades cellulose and hemicellulose into acetic, formic and other acids. These acids play an important preservative role but they also make the food taste acrid at high levels. High lignin woods require special attention since they burn too hot unless their combustion is slowed by restricted airflow or higher moisture.
Controlling the smouldering temperature is not only important for flavours, it influences the PAH concentration. Polycyclic Aromatic Hydrocarbons (PAHs) are formed in incomplete combustion processes, which occur whenever wood, coal or oil are burnt. Particular attention has been paid to the highly carcinogenic benzo[a]pyrene (BaP). A number of studies on smoked foods reveals that the highest levels of PAHs are found in products from traditional kilns that use smouldering wood or sawdust. In such kilns the combustion temperature is difficult to control and is usually very high. Toth and Blaas (1972) found that there is a linear rise in the concentrations of BaP and other PAHs in the smoke phase between smoke production temperatures of 400 – 1000 °C. These traditional techniques are often considered synonymous with ‘quality’ and ‘authenticity’…. so what should we do then? Eat industrially-smoked products because they are ‘more healthy’? My personal choice will always go toward taste but this does not mean we cannot adapt traditions to make the best of both kinds of knowledge. It has for example been proven that both cold-smoked and hot-smoked fish by an external smoke generator had lower PAH content. Moreover a Danish study reported that a cold-smoked fish has a lower BaP concentration then hot-smoked ones. In order to diminish the contamination of smoked products by PAH, the clear recommendation is to use indirect smoking, preferably cold smoke, and to maintain your smouldering temperature around 400°C.
These conclusions stimulated me to experiment a little.
Hot-smoked fishes are nowadays more popular in Scandinavia then cold-smoked ones, mackerel being the most consumed. How could we achieve this same taste without hot smoking the fish and keeping the PAH low? Hot-smoking occurs within the range of 52°C to 80°C. At this temperature food cooks while smoking. I therefore decided to cook the fish first in our combi oven and then cold-smoke it.

After a few experiments trying to find the best cooking temperature and humidity, here is the recipe:
Cold-smoked mackerel
For very fresh mackerel of around 400 g.
Gut them and brine them 8 h at 4°C in a solution of 20% salt of the total.
The aim is to reach a salt content of 3% in the fish … like the salinity of seawater in the Atlantic ocean…
Remove fish from the brine and place on a perforated tray.
Cook them in the oven at 70°C with 60% humidity.
Program your combi oven so when the core temperature reach 59°C the oven stops.
Take them out of the oven and cool them down in the blast freezer until they reach 4°C.
Cold smoke them for about 24h. We used beech wood but many other options are possible.
One of the great thing about this technique is that you can accurately control cooking temperature and humidity, things that are difficult with a hot-smoker. This control ensures the smoke flavours stay light and delicate, a taste certainly more suited to a contemporary palate.
Bibliography
Duedahl-Olesen, L.; White, S.; Binderup, M.L. Polycyclic Aromatic Hydrocarbons (PAH) in Danish Smoked Fish and Meat Products, Polycyclic Aromatic Compounds, Vol. 26, 3, 2006, p. 163-184
Knockeart, C., (1990), Le fumage du poisson, IFREMER
http://archimer.ifremer.fr/doc/00004/11490/8046.pdf
Mc Gee, H. (2004), Food & Cooking: an encyclopedia of kitchen science, history and culture, Hodder and Stoughton.
Myhrvold, N. and al. (2011), The modernist cuisine: the art and science of cooking, The cooking Lab, Vol 2, p 134 – 149.
Siesby Birgit, (1997), Scandinavian ways with fish, Fish: Food from Waters, Oxford Symposium, p 280 – 282
Toth, L., Blaas, W., (1972). The effect of smoking technology on the content of carcinogenic hydrocarbons in smoked meat products. Fleischwirtschaft, 52, 1419-1422.
www.thegoodsensecompany.com
